noh lewis structure
It was introduced in 1916 by Gilbert N. A nitrosyl chloride molecule consists of one atom of nitrogen one atom of chlorine and one atom of oxygen.

Lewis Dot Structures How To Calculate The Number Of Lone Pairs Using A Formula Youtube
Lastly there is a single unpaired electron on the nitrogen atom.
. First take a molecule of NOCl. Valence electrons of hydrogen 1. Oxygen has 6 and Fluorine has 7.
For a total of 18 valence electrons. Lewis introduced the concept of electron dot structure. As discussed earlier atoms are most stable when their octet is complete.
Follow some steps for drawing the lewis dot structure for NH2OH. Previous question Next question. Valence electrons of nitrogen 5.
Draw the lewis structure for Moscovium pentabromide. Part E NOH Draw the Lewis dot structure for NOH3. Within these noh can be divided into two categories genzai noh present time noh and mugen noh phantasmal noh.
Heres how you can draw the NO lewis structure step by step. Lets do the NOF Lewis structure. Salzsäure liegt in wässriger flüssiger Form ohne Farbe und mit stechendem Geruch vor.
Due to lone pair electron compression the H-N-H and H-N-C bonds angles are shorter than the standard 1095o. For the NH2OH Lewis structure calculate the total number of valen. Also there is an unpaired electron on nitrogen atom.
Exceptions are hydrogen and boron elements 2. Looking at the above structure it is clear that the octet of all the three atoms involved is satisfied and the lone pairs are placed as far apart as possible indicating this to be the most precise lewis structure for NOF. NOH3 is actually NH2OH.
Genzai noh are noh with stories happening in the present. NO2- is also known as nitrite ion. If you are a beginner to.
Follow the octet rule where an atom should complete its outermost shell by the total number of 8 electrons. Mark charges Step 4. A step-by-step explanation of how to draw the HNO Lewis Dot StructureFor the HNO structure use the periodic table to find the total number of valence electr.
There are 18 valence electrons in NO2- 9 electron pairs in NO2- 6 lone pairs. Put the Oxygen here and then the Fluorine on the other side. Nitrogen is the least electronegative thatll go in the center.
The double bar between the two chemical symbols means that nitrogen and oxygen share a double bond2 pairs of electrons. The NF3 is polar because NF3 has a lewis structure and. Mark lone pairs Step 3.
Lewis Structure of NOCl. Lewis structure of N OH3 N O H 3. Minimize charges Step 5.
The - symbol means that the molecule received an electron and became an ion. The macrocyclic structureis not only a breakthrough patent but also a problem-solving. The lewis structure of NH2OH has a total of 3 lone pairs and 4 bond pairs.
Recommended dose for non-small cell lung cancer 150 mg once daily on an empty stomach. This Lewis Structure contains a covalent bond between the Oxygen and Hydrogen in the hydroxide as well as an ionic bond between the Na 1. Lewis Structure For No2 In lewis structure NO 2- ion there are three lone pairs in the last shell in one oxygen atom and that oxygen atom is joint with nitrogen atom by a single.
Heres how you can draw the NOCl lewis structure step by step. Draw the lewis structure for OganessonOGdifluoride. NH2OH lewis structure has two N-H bonds one N-O bond and one O-H bond.
Count total valence electron in NH2OH. Is available as oral tablets each containing 25 mg 100 mg or 150 mg of erlotinib. The nitrogen exhibits a tetrahedral geometry because to the sp3 hybridization.
Lewis structure of NO. Hier diskutieren wir über die Lewis-Struktur die Eigenschaften und einige schnelle Fakten von HCl. Salzsäure HCl ist eine Flüssigkeit und Chlorwasserstoff HCl ist ein Gas.
Methane has 4 regions of electron density around the central carbon atom 4 bonds no lone pairs. The C-N sigma bond is formed when one of the sp3 hybridised orbitals collides with an sp3 hybridised orbital from carbon. Though perhaps confusing each of these types of noh have a system of.
Most stable lewis structure of NO is shown below. A Lewis structure for NO would look like. It packs itself among hydroxide ions OH -1 which are theoretically where sodiums electrons were donated to.
Well put two valence electrons between the atoms to form chemical. Lewis Structure Molecular Geometry. Lewis structure of NO 2 Nitrogen dioxide is drawn in this tutorial step by step.
In 1916 American chemist Gilbert N. Valence electrons of oxygen. While mugen noh have more complicated stores that involve dream states or visions intersecting with present time stories as well.
Below are some rules to frame any compounds Lewis dot structure. To do so have a look at the periodic table. Minimize charges again if there are Lets break down each step in detail.
Nitroxyl HNO CID 945 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists. NO2- Lewis Structure can be drawn easily if you know the valence electrons of NO2- total electron pairs of NO2- total lone pairs of NO2- and the charges of NO2-. Now we will find out the total number of valence electrons present in one NOCl molecule.
Steps of VSEPR rule to draw lewis structure of NO. Nitrogen on the periodic table is in group 5 or 15 so it has 5 valence electrons. Depending upon the number of atoms in a.
Chlorwasserstoff ist ein gelbliches brennbares Gas und von Natur aus korrosiv. There is a double bond between nitrogen and oxygen atom. View the full answer.
Sodium hydroxide is a sodium atom a metal having lost one electron to become an Na 1 ion. The nitrogen is the central atom and there is one lone pair on it. Nitric oxide is composed of a single nitrogen atom that is bonded to a nitrogen atom.
Draw sketch Step 2. Minimize charges again if there are Lets break down each step in detail. Following VSEPR rule steps are followed to draw the lewis structure of NO and they are explained in detail in next sections.
This is a special case of lewis structure drawing because there is a unpaired electron on nitrogen atom. The Lewis structure for NOF is. This is the best answer based on feedback and ratings.
Draw the lewis structure for Livermorium diiodide. A step-by-step explanation of how to draw the NO Lewis Dot Structure Nitric acidFor the NO structure use the periodic table to find the total number of va. Lewis Structure for NO 2 Nitrogen dioxide Oxidation number.
Include all lone pairs of electrons. You can learn basics of how to draw a lewis structure properly from this example. 50 What is the difference in length and strength of single bond vs One C atom is bonded to two H atoms and the other is bonded to an H atom and a Br atom Lewis who introduced it in his 1916 article The Atom and the Molecule Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent.
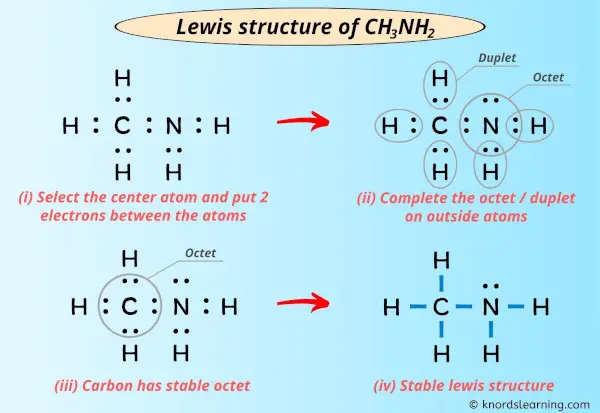
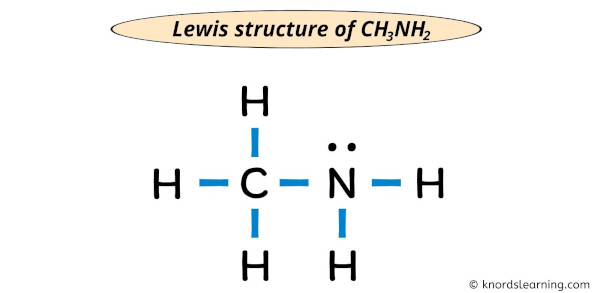
Lewis Structure Of Ch3nh2 With 6 Simple Steps To Draw

Lewis Structure Of Ch3nh2 With 6 Simple Steps To Draw

Nh2oh Lewis Structure How To Draw The Lewis Structure For Nh2oh Hydroxylamine Youtube

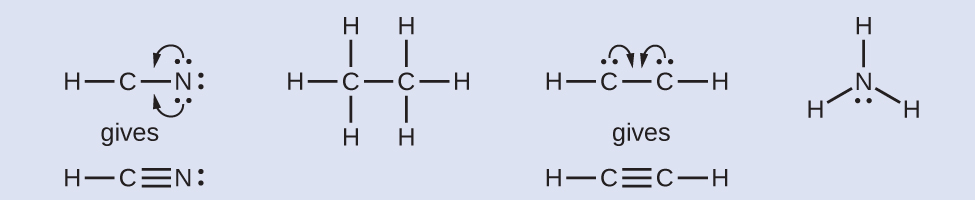
Hcn Lewis Structure Hydrogen Cyanide Molecules Lewis Biology

Nh2oh Lewis Structure Hydroxylamine Math Lewis Chemical Formula

N3 Lewis Structure Azide Ion Ap Chemistry Molecules Lewis

Lewis Dot Structure For Na2o Sodium Oxide Ionic Bonding Ionic Compound Ap Chemistry
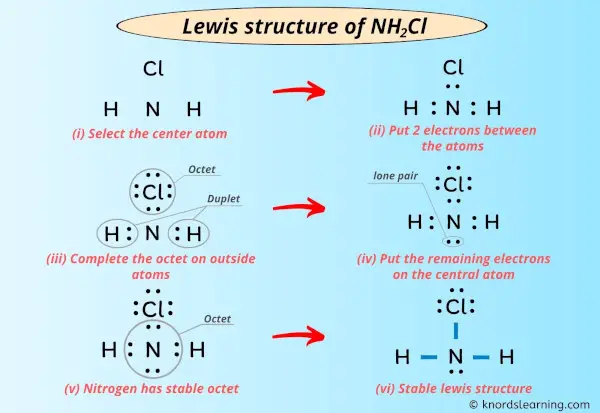
Lewis Structure Of Nh2cl With 6 Simple Steps To Draw

Nocl Lewis Structure Nitrosyl Chloride Math Lewis Molecules

Ch3oh Lewis Structure Methanol
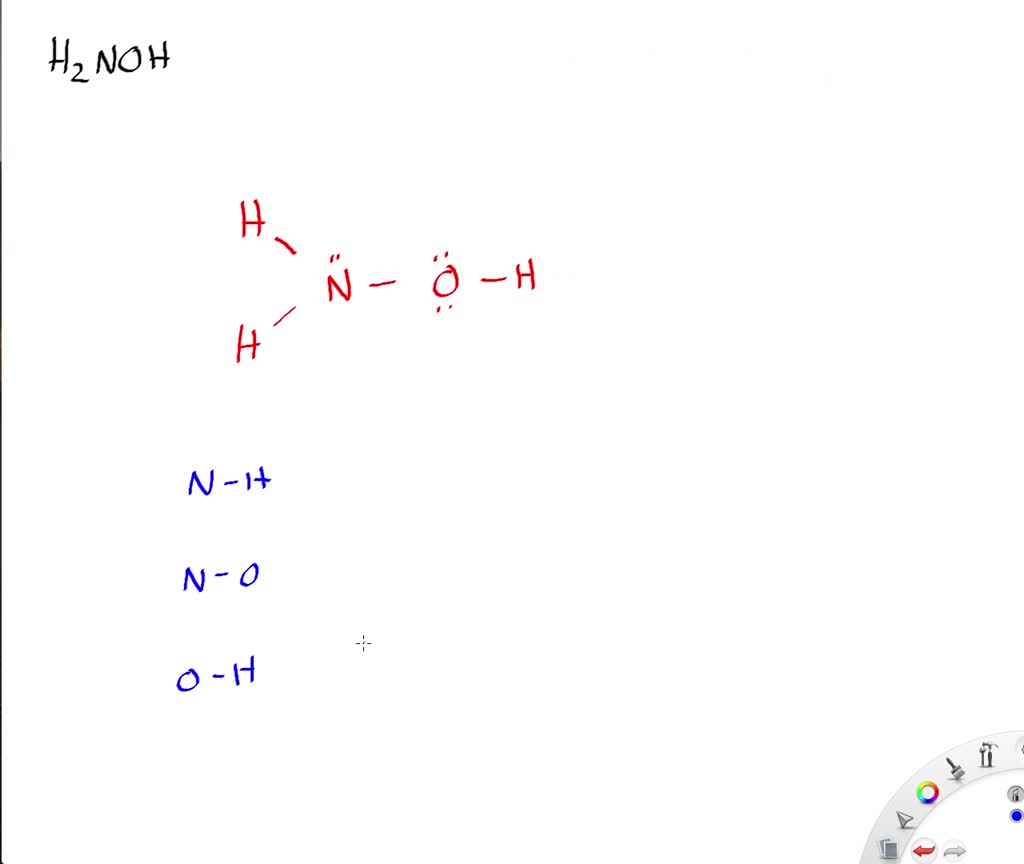
Solved Write A Lewis Structure Of The Hydroxylamine Molecule Mathrm H 2 Noh Then With Data From Table 10 2 Determine All The Bond Lengths

Lewis Structure Of Nh2cl With 6 Simple Steps To Draw

Nh2oh Lewis Structure How To Draw The Lewis Structure For Nh2oh Hydroxylamine Youtube
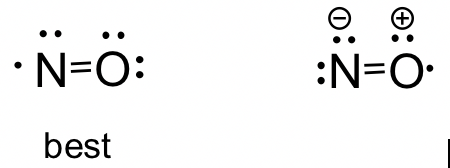
1 2 Lewis Structure Organic Chemistry I
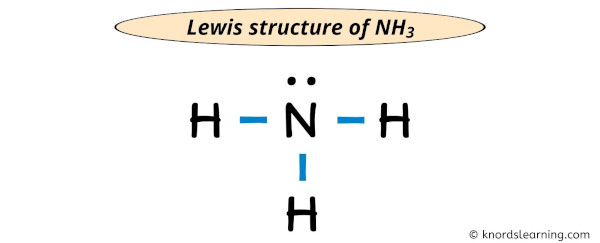
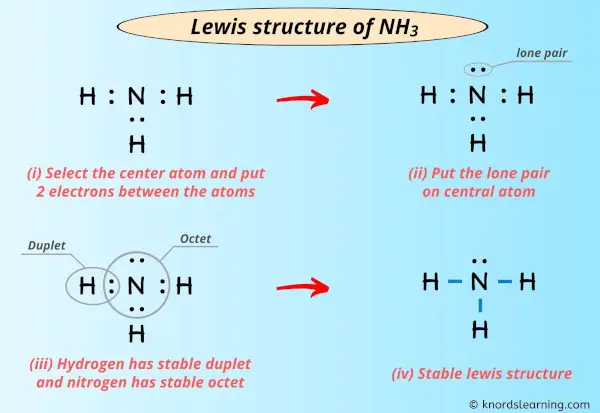
Lewis Structure Of Nh3 Ammonia With 6 Simple Steps


Comments
Post a Comment